British regulators have granted approval for the first-ever treatment derived from CRISPR, the groundbreaking gene-editing technique. The treatment, named Casgevy, is designed to address sickle-cell disease and the related condition, beta thalassemia. Vertex Pharmaceuticals, based in Boston, and CRISPR Therapeutics, based in Switzerland, are the manufacturers behind this innovative therapy. Approximately 2,000 patients in the United Kingdom with sickle-cell disease or beta thalassemia are expected to be eligible for Casgevy.
The companies anticipate approval from the U.S. Food and Drug Administration (FDA) for Casgevy in early December for sickle-cell patients in the United States. The decision on approval for beta thalassemia is expected to come next year. Another sickle-cell gene therapy, developed by Bluebird Bio of Somerville, Mass., is also anticipated to receive FDA approval in late December. It’s worth noting that Bluebird Bio’s treatment does not rely on gene editing but instead uses a method that inserts new DNA into the genome.
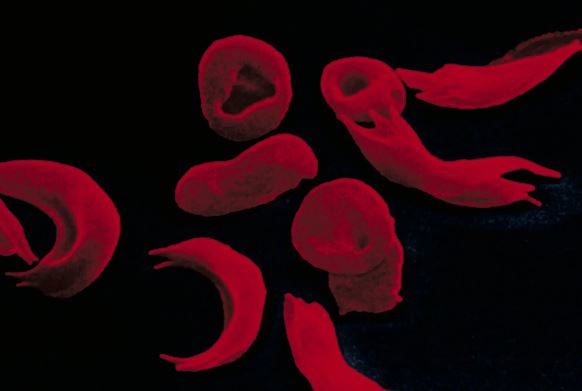
Sickle-cell disease is caused by a defective gene that results in the production of abnormal hemoglobin, affecting the oxygen-carrying function of red blood cells and causing episodes of severe pain. Approximately 100,000 Americans, mainly from Black and Hispanic communities, are believed to have this illness. In beta thalassemia, a faulty gene leads to insufficient levels of hemoglobin in red blood cells, and the condition is rare.
Casgevy utilizes CRISPR to edit the DNA, activating a gene that produces an alternative form of hemoglobin. To qualify for the sickle-cell treatment in Britain, patients must be at least 12 years old and have experienced repeated episodes of extreme pain. There is no upper age limit, and patients with organ damage from sickle-cell disease are not automatically excluded.
However, patients must have no other viable options for treatment. While sickle-cell disease can be cured with a bone-marrow transplant, finding compatible donors is a significant challenge. The Vertex and Bluebird treatments offer hope for those struggling with the disease, addressing not only pain but also complications such as bone and organ damage and strokes.
Despite their potential benefits, the CRISPR and Bluebird treatments are complex and demanding, requiring specialized expertise that many hospitals may lack. Patients undergo intensive chemotherapy to clear their bone marrow of abnormal stem cells, making room for genetically altered cells. Following this, patients must spend a month or more in the hospital while their marrow regrows.
Moreover, gene editing is an expensive process. While the price for Casgevy in Britain has not been disclosed yet, it is expected to be in the millions of dollars per patient in the United States. Sickle-cell disease itself is costly, estimated to cost the U.S. health system around $3 billion annually.
In the United States, Bluebird Bio has an approved gene therapy for beta thalassemia, priced at $2.8 million per patient. Vertex is currently testing its sickle-cell treatment in children aged 5 to 11, aiming to prevent irreversible organ damage over time.
The first patient to receive Vertex’s sickle-cell treatment, Victoria Gray, expressed that the therapy has transformed her life. Diagnosed with sickle-cell disease at three months old, Gray faced frequent pain crises and hospitalizations throughout her life. After receiving the gene editing treatment in 2019, she reported that all her symptoms have disappeared, describing it as a “new beginning” that surpassed her dreams.
As these innovative gene therapies pave the way for transformative treatments, the challenge lies in ensuring accessibility, managing costs, and building the expertise needed to administer these complex therapies effectively. The recent advancements in gene-editing technology offer hope for patients with genetic disorders, marking a significant milestone in the field of medicine.