According to a research that was released on Wednesday, an investigational medicine that was originally created to combat cancer was shown to lower the chance of mortality for those who were hospitalised with Covid by half.
The medication known as sabizabulin seemed to be more effective than the others that have been approved for use in individuals suffering from severe cases of Covid. The Miami-based business Veru, which was responsible for the development of the medication, has submitted an application to the Food and Drug Administration (FDA) for an urgent permission of its usage. According to the opinions of the many specialists, this has the potential to offer a new tool to the limited toolbox that is accessible to hospitalised patients.
However, Dr. Schwartz did point out that the experiment was on a rather modest scale, with just 134 individuals getting the medicine. “Overall, I believe this to be quite interesting; nevertheless, I would welcome bigger and independent investigations to validate it,” he stated. Sabizabulin prevents cells from constructing microtubules, which are essential molecular cables that move material throughout the inside of the cell from one location to another.
Researchers at the University of Tennessee first created the medicine with the intention of combating cancer due to the fact that rapidly dividing tumour cells are dependent on the microtubules for their rapid division. In an experiment that took place at Veru two years ago, sabizabulin was tested on Covid. They had a hunch that the medication may inhibit viral reproduction, as this process is dependent on the microtubule network in order to put together new viruses from their constituent parts.
They also had the hypothesis that the medicine might assist patients with Covid in the battle against lung inflammation, which may be potentially fatal. This immune response begins when cells realise they are contaminated and send alarm-signal proteins into their surroundings. This triggers other cells in the body to begin fighting the pathogen. In order for the message to be sent, the cells must first move the alarm molecules along their microtubules.
Early in the year 2020, researchers at the University of Tennessee Health Science Center discovered that sabizabulin might dampen the alarm signals that were being sent out by mouse cells. A few months later, Veru started evaluating the medicine in human subjects by having them take it in tablet form. In May of 2021, they moved on to the further stages of testing.
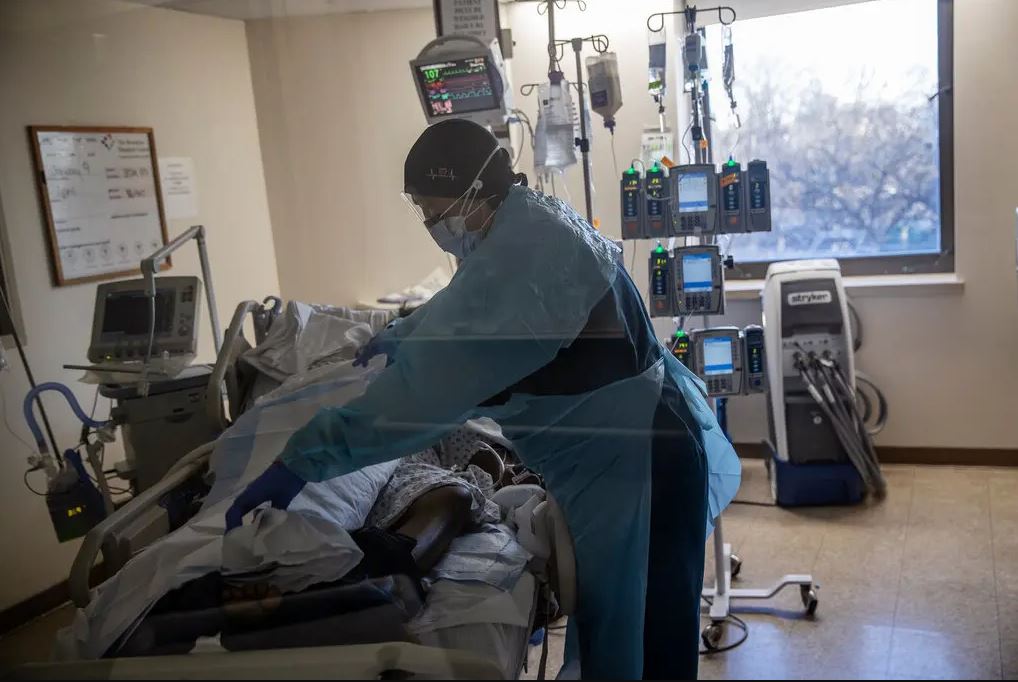
The corporation looked for volunteers among those patients who were already being treated at the hospital in order to test Covid. Patients needed to be dependent on a ventilator or be receiving oxygen in order to be considered for participation in the research. In addition to this, they needed to have a high probability of passing away from COVID due to the presence of risk factors such as hypertension, old age, or obesity. The patients were given permission to receive additional therapies at the same time, including those that have been shown to be useful in preventing death in hospitalised Covid patients. For instance, the steroid known as dexamethasone has been shown to cut the chance of passing away by one third.
In the most recent study, 70 subjects were given a placebo while 134 were given sabizabulin. The mortality rates of the two groups were drastically different after a period of 60 days: 45.1% of the people in the placebo group passed away, while only 20.2% of those who took the new medicine did so. This disparity resulted in a decrease in mortality risk that was 55.2 percent lower than expected.
A specialist in infectious diseases at the University of Minnesota named Dr. David Boulware issued a warning that the huge number of fatalities that occurred in the placebo group could be an indication that the research was too small to reach definitive findings. If administered to a patient at an early stage in the progression of their illness, a variety of antiviral medications have shown promise in reducing the likelihood that they may need hospitalisation as a consequence of their Covid infection. Paxlovid, for example, has been shown to cut the risk of hospitalisation for unvaccinated individuals who had Covid risk factors by around 90 percent.
These medications, on the other hand, do not function very well for hospitalised patients who have moderate to severe Covid. This is due to the fact that they are only capable of preventing viruses from replicating, rather than restraining an overactive immune response. There are fewer medications available for hospitalised patients, thus physicians have less of a selection to pick from. Tocilizumab, another anti-inflammatory medication, has been demonstrated to be helpful in addition to dexamethasone and baricitinib for treating this condition.
When Veru first announced its findings in April, the company stated that it had prematurely ended the trial because an independent advisory committee had determined that the benefits of sabizabulin were already evident from the data. The committee came to the conclusion that continuing to give some patients a placebo would be unethical.
Dr. Boulware was aware of the ethical considerations that the circumstance posed, but he also speculated that if the test had been conducted over a longer period of time, the advantages of the medicine could have turned out to be less impressive. Dr. Boulware observed that the anti-Covid medication molnupiravir had the appearance of initially lowering the risk of hospitalisation brought on by Covid by fifty percent. However, after further investigation, that percentage was found to be just 30%.