Dr. Michael Blaiss, an allergist at the Medical College of Georgia, has often been surprised by how hesitant his patients are to use their auto-injectors, such as the EpiPen, which deliver life-saving epinephrine to counter potentially fatal allergic reactions. Some patients, even on the brink of anaphylactic shock, call his office to confirm if using the device is necessary, while others prefer to wait in an emergency room parking lot, hoping their symptoms will subside without intervention.
The fear of needles, particularly among children, can be so intense that it leads to dangerous delays. Dr. Blaiss recalls instances where children, terrified of the injection, ran away from their parents, sometimes resulting in mishaps like a parent accidentally injecting themselves.
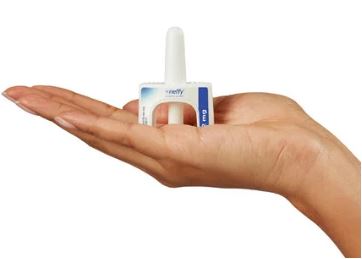
However, a new device recently approved by the Food and Drug Administration (FDA) may help ease this anxiety. The device, known as Neffy, administers epinephrine—the same drug found in the EpiPen—through a nasal spray rather than a needle. This innovation has been highly anticipated by both patient advocates and allergists who regularly witness the consequences of needle aversion.
Needle fear is widespread, affecting most children and up to 30 percent of young adults, according to a systematic review. Ilana Golant, founder and CEO of the Food Allergy Fund, noted that some parents have been so nervous about administering the shot that they missed the crucial window to prevent a serious reaction, leading to hospitalization for their children.
Dr. Jonathan Spergel, an allergist at the Children’s Hospital of Philadelphia, was so concerned about his patients’ reluctance to use needles in emergencies that he had them practice self-injecting in his office to show them it wasn’t as bad as they feared.
Neffy offers several advantages beyond addressing needle phobia. The nasal spray is small—just over two inches tall—making it more convenient to carry than the bulkier, six-inch EpiPen. Dr. Blaiss noted that many of his patients rarely carry two EpiPens, which is recommended in case the first dose is insufficient. With something as compact as Neffy, carrying two doses is much easier.
Richard Lowenthal, CEO of ARS Pharma, the developer of Neffy, stated that the device is expected to be widely available by the end of September and will be relatively affordable for most insured patients. The company plans to cap out-of-pocket costs at $25 for most people with commercial insurance and $199 for those with high-deductible plans or no insurance. Neffy’s shelf life of about two and a half years also means it may need to be replaced less frequently than an EpiPen, which typically expires 18 months after manufacture.
However, Neffy may not be suitable for everyone. Currently, it is only approved for adults and children over 66 pounds, although the company expects it to be available for children over 33 pounds by next summer. Additionally, some patients may prefer to stick with the tried-and-true EpiPen or its generic versions due to the limited clinical data on Neffy’s effectiveness during actual anaphylactic episodes.
Clinical trials for severe allergic reactions are ethically challenging, as researchers cannot induce life-threatening reactions and then administer a placebo to half the participants. Instead, the efficacy of Neffy was tested on healthy subjects, with researchers comparing their reactions to those of participants who received epinephrine injections. The studies showed that the concentration of epinephrine in the blood, heart rate, and blood pressure—all indicators of drug absorption—were consistent between the two groups, suggesting that the spray is similarly effective.
Researchers also conducted trials on individuals with nasal congestion and found similar results. However, Dr. Blaiss emphasized the need for caution, recommending that patients who have experienced severe allergic reactions carry an EpiPen as a backup until more real-world data on Neffy becomes available.