According to draught guidance provided by the Food and Drug Administration, doctors are required to inform patients who are considering undergoing Lasik surgery that they run the risk of being left with double vision, dry eyes, difficulty driving at night, and, in extremely rare instances, ongoing eye pain. The statement cautions that even after surgery, individuals could still need the use of spectacles.
Many people in the United States may be taken aback by the cautions issued by the government agency since they consider the practise to be risk-free and standard operating procedure. In the United States alone, more than half a million adult patients have Lasik surgery each year to treat vision problems.
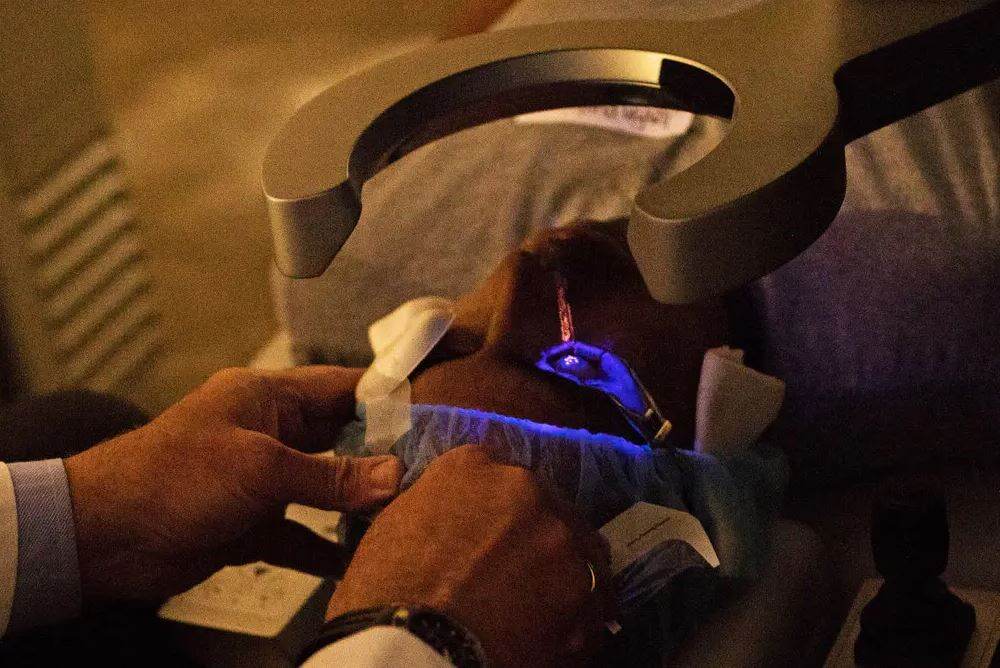
Recontouring the cornea, the transparent, dome-like structure that covers the front of the eye and directs light to the retina at the rear of the eye, is one of the tasks included in the procedure. After making a small incision in the cornea and folding it over, the surgeon will use the laser to sculpt the cornea into the desired shape. After that, the flap will be reattached by the surgeon.
Patients must often spend thousands of dollars out of pocket for the treatment, which is not covered by insurance since it is deemed cosmetic. The operation typically lasts less than 15 minutes for each eye, and patients must pay for it themselves. Lasik physicians often market the operation by providing free consultations and substantial discounts. These surgeons refer to statistics indicating that 90 to 95 percent of consumers are happy with the surgery.
The material pertaining to the FDA is not yet complete. Since the draught of the advice was made public in July, more than 600 people and professional organisations have provided feedback in the form of comments. The agency is now examining the information while drafting the final materials, according to authorities.
Organizations that represent surgeons and manufacturers of medical devices have taken the offensive, accusing the FDA of interfering with the practise of medicine and stating that the information is one-sided and will unnecessarily scare patients. These organisations say that the FDA is meddling in the practise of medicine.
The majority of ophthalmologists agree that Lasik is the safest treatment that can be performed on the eye, and they also agree that significant consequences that last a lifetime are quite uncommon.
Some patients claimed in their remarks to the agency that they had life-changing consequences and visual loss, while others were ecstatic with the outcomes of their treatment.
The professional organisation that represents optometrists, who conduct vision tests and prescribe eyeglasses and contact lenses, praised the draught and recommended adding even more precautions about Lasik surgery for pregnant patients and those with irregular astigmatism. Optometrists perform vision tests and prescribe eyeglasses and contact lenses.
On the other side, the Medical Device Manufacturers Association has accused the Food and Drug Administration of exceeding the scope of its power and “illegally controlling the practise of medicine.”
Officials from the agency shot off the complaint in an email response, stressing that the Food and Drug Administration (FDA) frequently issues labelling recommendations in order to ensure that both physicians and patients understand the advantages and hazards of medical devices.
Breast implants were given what is known as a “black box warning” and a new label by the Food and Drug Administration (FDA) last year. The FDA also mandated that health care practitioners discuss possible dangers, such as cancer, with prospective patients.
In 2018, the government placed restrictions on the sale of a permanent contraceptive implant known as Essure, requiring that physicians go through a list of safety issues with patients before prescribing the device.
The first study found that three months after undergoing Lasik surgery, nearly half of patients who did not experience any visual symptoms prior to the procedure developed a new visual aberration for the first time. The most common visual aberration that patients experienced was halos, which are starburst-like shapes that appear around lights. At three months, over one third of patients reported having dry eyes.
Patients who undergo Lasik surgery should be adequately counselled about the possibility of developing new visual symptoms after surgery. Despite this, over ninety percent of patients reported being pleased with the result of their treatment.
The organisation carried out its own research, which revealed that six months following surgery, 2 percent of patients had issues that prohibited them from partaking in their typical daily activities, and 27 percent of patients reported dry eyes.
According to the Food and Drug Administration, five years after surgery, 17 percent of patients still relied on eye drops, 2 percent continued to experience visual disturbances, and 8 percent still had difficulty driving at night. All of these statistics are based on patients who underwent cataract surgery.
Officials from the F.D.A. said that they did not have any information on when the guidelines will be completed. Because the government is not mandating a review prior to surgery, critics of Lasik are worried that a large number of patients will not have the opportunity to see the warnings before undergoing the procedure.
The instructions and the checklist “should be offered by manufacturers and presented to doctors and patients before a treatment,” and they should be used to “improve” the dialogue between the physician and the patient about the risks and advantages of the surgery.
However, representatives from the organisation recognised that sharing the information with patients is merely a guideline. According to Diana Zuckerman, director of the National Center for Health Data, a nonprofit think tank that studies research on medical and other consumer items, this is a problematic statement.