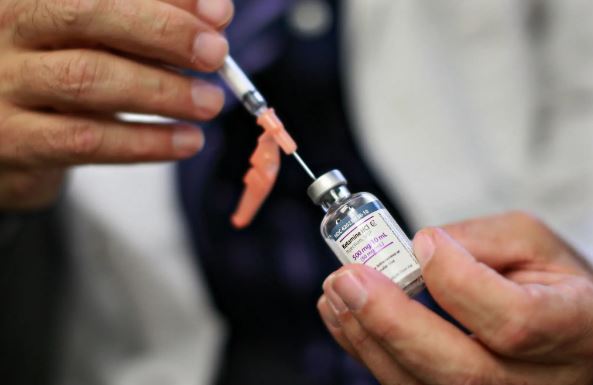
On Tuesday, the FDA issued a warning about the risks of using compounded forms of ketamine to treat psychiatric disorders. Ketamine is a potent anaesthetic that has gained popularity as an alternative treatment for depression, anxiety, post-traumatic stress disorder, and other challenging mental health issues.
Drugs that have been “compound” for a specific patient are those that have been altered or customised in a laboratory setting.
The FDA issued a warning about the dangers of using compounded ketamine without supervision, citing reports it had received of adverse incidents such as increased blood pressure, respiratory depression, and urinary tract problems that can lead to incontinence.
The alert aimed to clarify the difference between ketamine’s controlled usage as a mental therapy provided in clinics and “wellness centres,” and the unsupervised use of ketamine sold online by marketers who prescribe the medication via telemedicine so that purchasers can take the drug in the comfort of their own homes.
Although ketamine was initially licenced for use as a battlefield anaesthetic in 1970, it has also acquired appeal as the snortable party drug Special K. Recently, there has been a surge in the use of injectable ketamine in the treatment of mental health issues that have proven resistant to conventional methods of care.
However, growing misuse is a consequence of the absence of regulations. Heavy, long-term use of ketamine can cause serious health issues, including permanent damage to the urinary system, and is also highly addictive.
The proliferation of telemedicine due to recent pandemics has given birth to a slew of internet prescribers who, after a short video chat, would provide low-cost ketamine lozenges, pills, or nasal sprays. Experts warn that firms who provide up to 30 dosages in a single session are encouraging abuse.
However, executives in the pharmaceutical compounding sector have voiced worry that state regulators, who have jurisdiction over the nation’s compounding facilities, may be overly strict if the F.D.A.’s guidelines is not sufficiently nuanced.
There was no information on ketamine users’ adverse effects in the F.D.A. warning. One patient with post-traumatic stress disorder who took oral ketamine compounded at home was reported to have had respiratory depression in April. According to the agency, the patient had double the quantity of ketamine in their system as is considered safe for anaesthesia.
Dr. Steven Radowitz, chief medical officer of Nushama, a NYC ketamine facility that delivers the medication through injection, expressed his hope that the advisory will assist patients in distinguishing between online vendors of the drug and clinics that provide treatments under close medical supervision. According to him, the treatment programme at Nushama consists of six ketamine sessions spread out over the course of three weeks and is administered by a team of in-house medical professionals.