As extreme heat continues to envelop much of the United States, an unexpected threat is emerging: the risk of prescription medications being damaged during transit due to overheating. Millions of Americans rely on mail-order pharmacies for their prescriptions, a practice that has grown increasingly popular due to convenience and insurance mandates. However, soaring temperatures inside delivery trucks, which can reach up to 150 degrees Fahrenheit, far exceed the recommended temperature range of 68 to 77 degrees for safe drug storage.
Mail-order pharmacies claim their packaging is weather-resistant and that they take special precautions for medications requiring specific temperature control. However, a 2022 study by independent researchers revealed a troubling reality. They found that packages containing simulated medications spent more than two-thirds of their transit time outside the appropriate temperature range, regardless of the shipping method, carrier, or season. This raises concerns about the integrity and effectiveness of many medications.
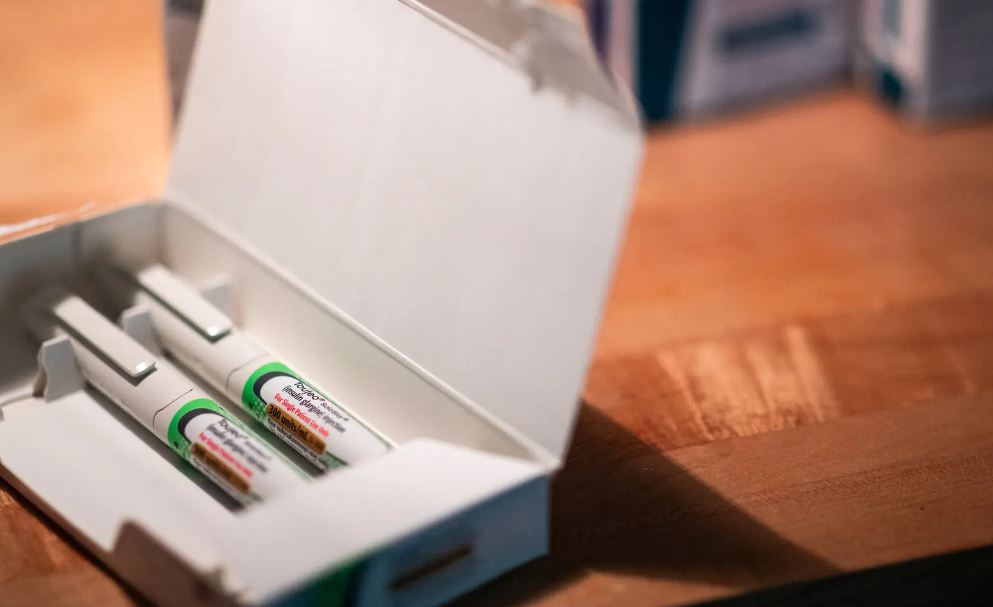
Certain medications are particularly vulnerable to heat. Liquid medications like insulin and AUVI-Q, an epinephrine injection used for allergic reactions, are at a heightened risk of degradation. Excessive heat can cause the liquid components, which are compounded at precise ratios, to evaporate or break down. Similarly, aerosolized medications face unique risks due to pressure changes in their canisters. These medications, which are often life-saving, need to work immediately, making their potential degradation even more concerning.
Despite these risks, some major mail-order pharmacies do not actively monitor the temperatures inside packages during transit. For instance, OptumRx, a major pharmacy benefit manager, uses predictive analytics and packaging innovations to protect medications but has discontinued the use of temperature-monitoring tags due to a high rate of false positives. Other companies, like Express Scripts, do not use special packaging or temperature tags for most room-temperature shipments.
The Food and Drug Administration (FDA) provides strict guidelines for packaging, storing, and transporting drugs between manufacturers, wholesalers, and pharmacies. However, these rules do not extend to the transportation of medications to patients. The FDA advises avoiding exposure of medications to extreme temperatures, warning that drugs not stored properly may not work as well or could cause harm, even if they do not show visible signs of degradation.
Patients across the country have reported receiving medications they suspect were damaged by heat, sometimes with severe health consequences. For example, Loretta Boesing from Missouri received a shipment of liquid immunosuppressant medication for her son in 102-degree weather. The medication arrived in only a plastic envelope, and soon after, her son’s health declined, leading to a two-week hospital stay. Boesing later started a nonprofit, Unite for Safe Medications, to advocate for safer delivery practices.
Rebecca Nierengarten of Maplewood, Minnesota, experienced similar issues with her mail-order medication. After being forced to switch from hospital infusions to home delivery of an immunoglobulin product, she noticed that her condition worsened each summer. The heat-sensitive medication, which is a protein, likely degraded during transit, exacerbating her autoimmune disease.
In addition to heat, extreme cold can also damage medications. Cheri Hicks, an interior designer in Michigan, found that her blood sugar spiked unexpectedly after using mail-order insulin that had been delivered three days late due to a snowstorm. Her blood sugar returned to normal after switching to a sample insulin pen from her doctor, suggesting that the mail-order insulin had likely been damaged by freezing temperatures.
Efforts to regulate the temperature at which medications are transported have faced significant opposition. In Oklahoma, the state pharmacy board attempted to implement stricter rules requiring that medications be kept within the recommended temperature ranges. However, pharmacy benefit managers and their lobbyists successfully argued that the proposed regulations were unnecessary and would drive up costs. As a result, the rule was abandoned.
The risks associated with improper medication storage during transit are well-documented, yet regulatory action has been slow. Experts warn that it may take a significant tragedy to prompt meaningful change. Until then, patients are left to navigate the risks on their own, often without adequate information or support from the companies responsible for delivering their life-saving medications.