Philips Respironics made a significant announcement on Monday, revealing its decision to cease the sales of all its breathing machines in the United States. This move comes in the wake of a settlement reached with the Food and Drug Administration (FDA) regarding persistent issues with the devices.
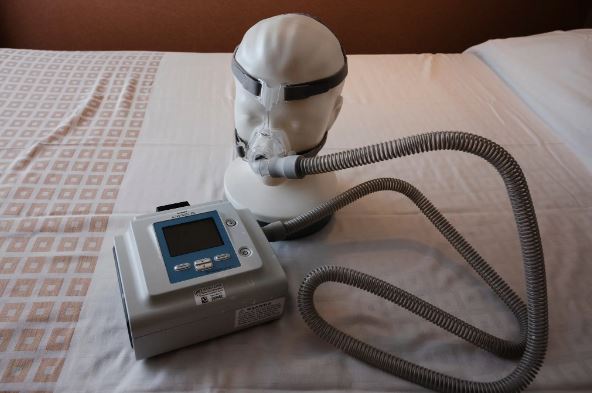
The company’s ventilators and CPAP (Continuous Positive Airway Pressure) machines, which aid in nighttime breathing, faced recalls due to reports indicating that they emitted foam particles and potentially harmful gases into users’ airways.
As part of the settlement, Philips has committed to meeting a series of standards outlined in a “multiyear” plan before it can resume operations in the U.S. Further details regarding the agreement will be disclosed upon its finalization in court. However, Philips assured that it would continue to service existing devices and provide support for users currently relying on them.
The recall process initially began in June 2021, with millions of devices affected. Sales of new sleep therapy machines to the U.S. were paused at that time, citing concerns over potential serious injury or long-term harm from the emission of potentially carcinogenic chemicals.
Subsequently, Philips conducted additional testing and released results indicating that the devices were not expected to cause significant harm to users’ health. However, the FDA questioned some of the company’s updated claims, labeling them as “unpersuasive.” Philips has also faced ongoing scrutiny and initiated further recalls as part of its efforts to address the issues with the devices.
Dr. Jeff Shuren, director of the FDA’s device division, refrained from commenting until the agreement is finalized and filed with the court.
The initial recall impacted approximately 15 million breathing machines manufactured since 2006, with around five million still in circulation as of mid-2021. The absence of immediate replacements caused confusion and distress among healthcare providers and patients alike, who grappled with the dilemma of using faulty devices versus risking compromised breathing during sleep.
Sleep apnea, characterized by interrupted breathing during sleep, affects millions of individuals and is associated with increased risks of strokes, heart attacks, and cognitive decline. The recalled machines included CPAP, BiPap, and ventilator devices.
Philips, headquartered in Amsterdam, disclosed the agreement, termed a consent decree, alongside its fourth-quarter earnings announcement. The company revealed a write-down of approximately 363 million euros related to the costs associated with fulfilling the settlement requirements. Consequently, its stock, traded in the U.S., experienced a decline of about 7 percent on Monday morning.
While Philips has halted sales in the U.S., it will continue to market its products in other countries.
Following the recalls, thousands of patients filed lawsuits against Philips, alleging a range of respiratory and other health issues, including claims of lung cancer-related deaths. In September, the company reached a $479 million settlement with plaintiffs to address financial losses incurred in repairing or replacing the machines. Litigation concerning illnesses and medical expenses remains ongoing.