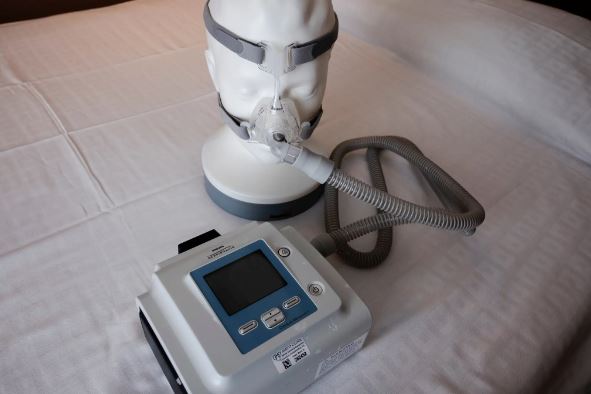
On Thursday, attorneys for the lawsuit’s plaintiffs announced that Philips Respironics had agreed to a $479 million partial settlement over claims involving the company’s breathing machines that spewed gases and flecks of foam into the airways of consumers, prompting recalls involving millions of the devices.
According to the attorneys, this settlement is only one part of ongoing class action litigation regarding the devices, and it solely addresses monetary refunds to device users and merchants who may have funded replacements for customers. There is no upper limit on the value of economic claims, so anybody who uses the gadget may file for reimbursement.
Other key issues in the plaintiffs’ suits, including personal damage and medical costs linked to use of the breathing equipment, are not addressed by this proposed settlement, which is subject to approval by the federal court. As part of the proposed settlement, Philips did not acknowledge wrongdoing or culpability.
After starting recalls in the US of nearly 5 million breathing equipment meant for individuals with sleep apnea and other ailments, the firm has been struggling for years. The complaints assert that respiratory diseases, lung cancer, and even death may be traced back to the machines’ off-gassing of flaky foam and gas. In order to lessen mechanical noise and vibration, the machines used foam.
The FDA issued a recall in June 2021 for Philips equipment, including BiPAP devices and ventilators, manufactured after 2009 because to concerns that foam breakdown in the goods might cause “serious injury” to users. Initially, Philips sent a statement to physicians expressing worry that the breakdown of the foam might have “toxic carcinogenic effects,” but the firm has subsequently issued revisions showing a far lower degree of concern.
The plaintiffs’ attorneys issued a statement saying, “We are confident in these claims and we look forward to holding Philips accountable for the physical harms they caused patients.”
Sleep apnea affects millions of individuals and is linked to breathing disruptions during sleep, putting them at risk for cardiovascular problems and cognitive impairment due to low blood oxygen levels.
Doctors and patients alike have been left wondering whether or not to seek treatment in light of the recent rash of recalls, given the risks associated with continuing to use the recalled devices. Many customers were left without an alternative because competing businesses were unable to meet demand.
According to the terms of the deal announced on Thursday, consumers would get anything from $50 to $1,500 in compensation, plus an additional $100 for each item that is sent back to Philips. Nearly 2.5 million gadgets were reportedly replaced and sent to customers and vendors in the United States.
The Food and Drug Administration and various experts have called out Philips for failing to alert customers as soon as the company became aware of problems with its products. Concerns at Philips first surfaced in 2015, according to agency and court filings. The F.D.A. has received reports of more than 105,000 injuries and 385 fatalities that may be connected to the foam breakdown in Philips equipment.
Philips said in its July results announcement that the U.S. Department of Justice had contacted the business regarding the possibility of a consent order to resolve issues with the recall process. According to the July report, Philips continues to comply with a subpoena filed in April 2022 as part of another inquiry into the circumstances behind the recall.