The severity of the condition may vary, but it can have a profound impact on some people’s lives. It can cause a complete loss of body hair, including eyelashes and eyebrows, as well as hair in the nose and hair in the ears. And up until very recently, there was no therapy available for alopecia areata patients that would cause their hair to come back.
Baricitinib, a medication produced by Eli Lilly that promotes the growth of new hair by preventing the immune system from attacking hair follicles, was granted approval by the Food and Drug Administration (FDA) on Monday. JAK inhibitors are the name given to a class of drugs that are being developed by two additional pharmaceutical companies, Pfizer and Concert Pharmaceuticals. Rheumatoid arthritis and other autoimmune illnesses may now be treated with these medications, which are currently available on the market. The clearance of these pricey pharmaceuticals by the F.D.A. is necessary for insurance companies to pay their costs, which average about $2,500 per month on the list price.
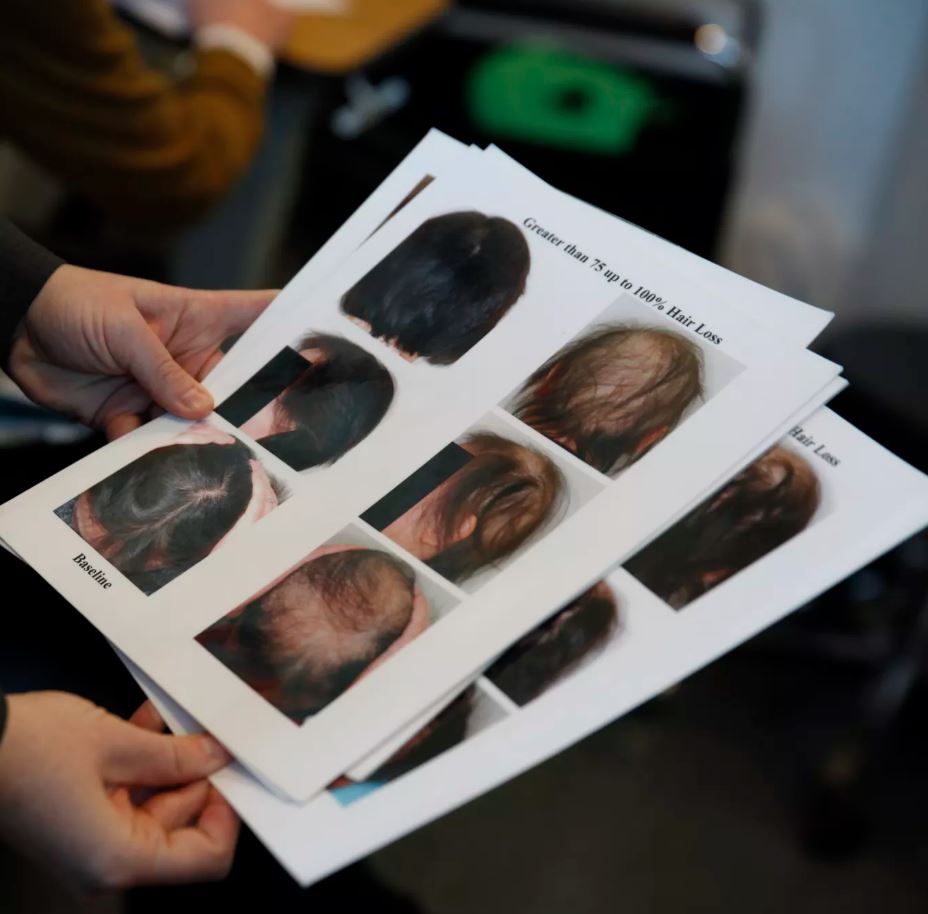
One thousand two hundred people suffering from a severe form of alopecia areata took part in the research for the medicine manufactured by Lilly that was funded by the firm and published in the New England Journal of Medicine just this past month. After a period of 36 weeks, almost forty percent of those who took the medicine saw full or nearly full regrowth of their hair. Nearly half of the patients had their hair growing back after a year of treatment.
Dr. Brett King, who teaches dermatology at Yale University and served as the principle investigator for both of the Lilly clinical studies, is also conducting clinical investigations that are being funded by the other firms. He expressed his optimism that the success rate of the medications will continue to improve in the future. It’s possible that the JAK inhibitors used to treat alopecia areata may be improved by the manufacturers. Patients who do not react to a medicine manufactured by one of the three firms may respond favourably to a treatment manufactured by another of the three companies once all three companies have pharmaceuticals available on the market.
Patients who participated in the Lilly trial were shown to have mild to moderate adverse effects, such as an elevated but still manageable risk of acne, urinary tract infections, and other infections. These adverse effects were readily treated or improved even in the absence of medical intervention.
Dr. Andrew Messenger of the University of Sheffield and Matthew Harries of the University of Manchester noted in an accompanying editorial that the findings of the Lilly study “are outstanding.” The researchers went on to say that these results “are the first reported phase 3 trials of any therapy for this illness.”
According to the Food and Drug Administration, more than 300,000 people in the United States live with a severe form of alopecia areata. According to Dr. King, it is difficult to overestimate the effect of the condition.
The condition known as alopecia areata often presents itself on the scalp of the affected individual as a single or a few discrete bald spots. However, people who have more severe instances are dealing with something far more serious. They may one day find that their heads have developed a few little bald patches. After three months, or perhaps three weeks, they are completely bald all over their bodies.
Alopecia areata is a skin condition that may be treated with medications called JAK inhibitors. Dr. King is largely acknowledged by his peers as being the one who first sparked interest in this treatment option. He said that everything got started when he came across three presentations that were presented at medical events in 2012 and 2013. Although the research was conducted on mice, it suggested that JAK inhibitors could be able to reverse alopecia areata. The research was headed by Dr. Raphael Clynes and Dr. Angela Christiano of Columbia University.
Soon after that, a guy called Kyle, who was 25 years old at the time, went to visit Dr. King in order to have his psoriasis treated. Psoriasis had left him with large, scaly plaques all over his head and body, and he had practically no hair.
When Kyle was in high school, he was attending a dance while wearing a hat, and that was when he first became aware that he was losing a significant amount of hair. He went to the restroom, removed his hat, and, to his astonishment, discovered a significant quantity of hair trapped within the hat.
He gave Kyle a thoughtful look and then said, “If you want to attempt something crazy that has never been done before, there is a drug that is authorised for rheumatoid arthritis and is being developed for psoriasis. There is some evidence in mice to indicate that it might be successful.”
Dr. Natasha Atanaskova Mesinkovska, who is also a dermatology professor at the University of California, Irvine, assisted pharmaceutical firms in recruiting patients for their clinical studies. Dr. Mesinkovska is the chief scientific officer of the National Alopecia Areata Foundation. She, too, has been pleased by the effects that have been seen in those individuals who have reacted well to the medications.
Brooke Nelson, a patient of Dr. Craiglow’s who resides in Belleville, New Jersey, had complete baldness when she was in the first grade. Her hair had been long and blonde. Because Brooke Nelson was so self-conscious about her thinning hair, her mother Danielle Nelson decided to educate her at home.
She brought Brooke to many medical facilities and numerous doctors, but none of the treatments or diagnoses were successful. According to Ms. Nelson, “I would have given up my home and given up everything if it meant giving Brooke back her hair.”
When Ms. Nelson discovered Dr. Craiglow, she was prepared to send Brooke there for stem cell treatment. Instead, Dr. Craiglow administered a JAK inhibitor to Brooke. Her hair began to return.